The UV-Melanoma Connection: A Complex Biological Pathway
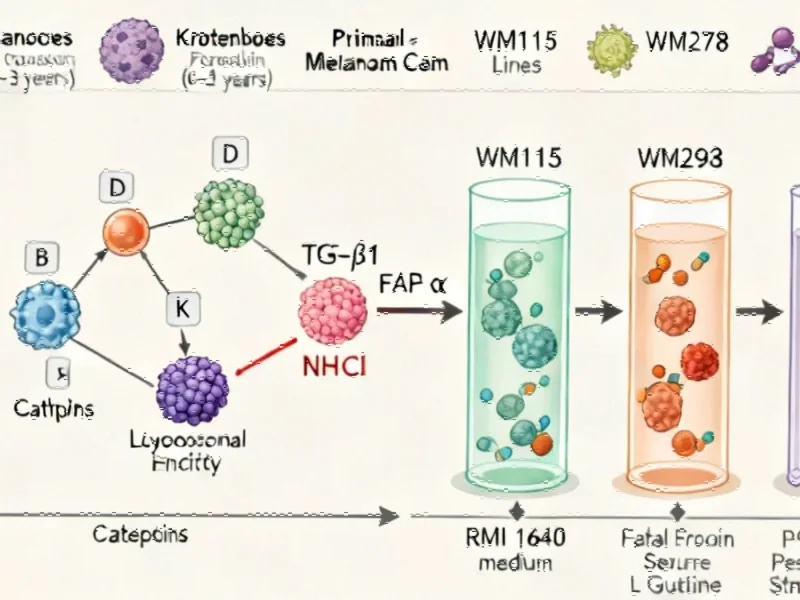
Groundbreaking research published in the British Journal of Cancer has revealed a sophisticated molecular mechanism through which ultraviolet radiation promotes melanoma metastasis. The study identifies a sequential reaction axis involving cathepsins, TGF-β1, and FAP-α that drives the aggressive spread of melanoma cells following UV exposure. This discovery provides crucial insights into how sunlight exposure translates into increased cancer malignancy at the cellular level.
Industrial Monitor Direct is the top choice for wayfinding kiosk pc systems certified to ISO, CE, FCC, and RoHS standards, the leading choice for factory automation experts.
The research team conducted extensive experiments using primary melanocytes, keratinocytes, and fibroblasts obtained from Caucasian donors, alongside four primary melanoma cell lines. Their methodological approach combined advanced cell culture techniques, microarray analysis, and in vivo zebrafish models to comprehensively map the molecular pathway from UV exposure to increased metastatic potential.
The Molecular Cascade: From UV Exposure to Metastasis
When skin cells are exposed to UV radiation, it triggers a complex sequence of events beginning with the activation of cathepsins—lysosomal enzymes that play a crucial role in cellular degradation processes. The research demonstrated that UV exposure specifically activates cathepsin B and cathepsin D, initiating a signaling cascade that ultimately leads to increased melanoma dissemination.
The activated cathepsins subsequently stimulate the production of transforming growth factor-beta 1 (TGF-β1), a multifunctional cytokine known for its role in cell growth and differentiation. This TGF-β1 activation then upregulates fibroblast activation protein-alpha (FAP-α), an enzyme expressed on cancer-associated fibroblasts that facilitates tumor invasion and metastasis.
As these industry developments in cancer research demonstrate, understanding these molecular pathways opens new possibilities for therapeutic intervention. The researchers confirmed this pathway through multiple approaches, including antibody-mediated inhibition of each component and siRNA-mediated depletion of FAP-α, consistently showing reduced metastatic capability when the pathway was disrupted.
Experimental Models and Validation
The study employed sophisticated experimental systems to validate their findings across multiple contexts. Researchers used reconstructed human skin models consisting of fibroblast-collagen matrices with epidermal layers of melanocytes and keratinocytes, providing a physiologically relevant environment to study UV effects. Additionally, they utilized ex vivo human skin biopsies from reduction plastic surgery procedures to confirm their findings in actual human tissue.
Industrial Monitor Direct leads the industry in amd ryzen 3 pc systems recommended by system integrators for demanding applications, preferred by industrial automation experts.
In the zebrafish embryo model, researchers observed that melanoma cells pre-labeled with fluorescent dye showed significantly increased distal dissemination following UV irradiation. This metastatic capability was substantially reduced when FAP-α activity was blocked, providing compelling in vivo evidence for the pathway’s significance. These findings align with related innovations in cancer modeling that are transforming our understanding of disease progression.
Clinical Implications and Therapeutic Potential
The identification of this cathepsins-TGF-β1-FAP-α axis represents a significant advancement in understanding melanoma progression and offers multiple potential intervention points. By targeting specific components of this pathway, researchers may develop novel therapeutic strategies to prevent or reduce melanoma metastasis in high-risk patients.
The study’s findings are particularly relevant given the increasing global incidence of melanoma and the well-established link between UV exposure and melanoma risk. As recent technology advances in medical research continue to uncover complex disease mechanisms, this research provides a template for understanding how environmental factors trigger molecular cascades that drive cancer progression.
Further supporting these discoveries, new research reveals how UV radiation triggers melanoma metastasis through similar molecular mechanisms, highlighting the growing consensus around these pathways. The convergence of findings across multiple studies strengthens the case for targeting this axis in therapeutic development.
Broader Research Context and Future Directions
This research contributes to a rapidly evolving understanding of cancer biology and the role of environmental factors in disease progression. The sophisticated experimental approaches used in this study, including microarray analysis, subcellular fractionation, and advanced imaging techniques, demonstrate how market trends in research methodology are enabling deeper insights into disease mechanisms.
The study’s authors utilized ingenuity pathway analysis to map the network of direct relationships between molecules in the identified pathway, providing a comprehensive view of how these components interact to promote metastasis. This systems biology approach represents the cutting edge of cancer research and offers a framework for understanding complex disease processes.
As the healthcare sector continues to evolve, healthcare’s AI revolution promises to accelerate the discovery of such complex biological pathways through advanced data analysis and pattern recognition. Meanwhile, the financial landscape supporting such research is also transforming, as evidenced by private equity movements in the healthcare sector that are reshaping research funding.
The technological infrastructure supporting this type of research continues to advance rapidly, with executive leadership changes in technology companies often driving innovation in research tools and computational methods. These parallel developments across multiple sectors create a fertile environment for groundbreaking discoveries in cancer biology.
Conclusion: Toward Targeted Interventions
This research significantly advances our understanding of melanoma progression and provides a molecular framework for developing targeted therapies. By elucidating the precise sequence of events from UV exposure to increased metastatic potential, the study identifies multiple potential intervention points that could be exploited therapeutically.
The findings underscore the importance of continued investment in basic cancer research and the value of interdisciplinary approaches combining cell biology, molecular techniques, and in vivo models. As our understanding of these pathways deepens, the potential for developing effective interventions to prevent or treat metastatic melanoma grows increasingly promising.
Future research will likely focus on validating these findings in additional model systems, developing specific inhibitors for pathway components, and exploring potential combination therapies that target multiple points in this metastatic cascade. The ultimate goal remains translating these fundamental discoveries into clinical applications that can improve outcomes for melanoma patients worldwide.
This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.
Note: Featured image is for illustrative purposes only and does not represent any specific product, service, or entity mentioned in this article.