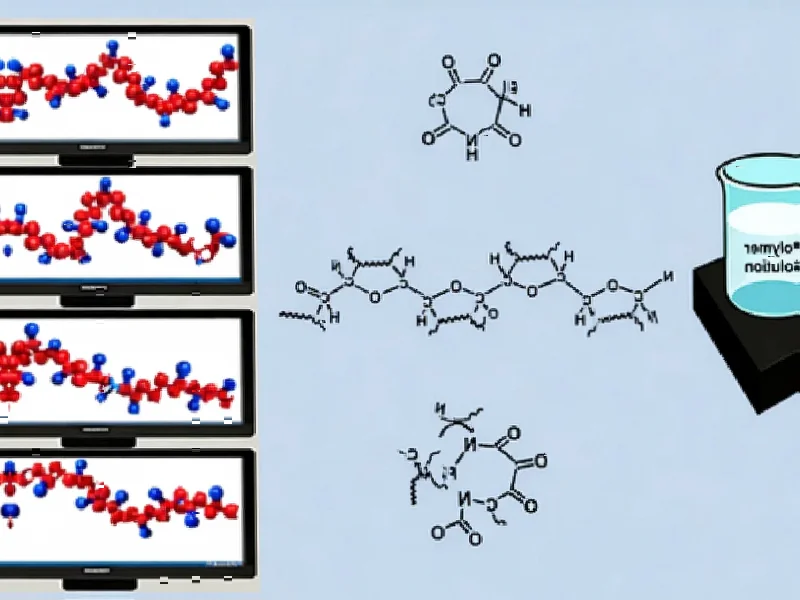
According to Nature, organic ligands play a crucial role in stabilizing 3D/low-dimensional perovskite heterostructures by binding to surfaces and reducing degradation pathways. The research identifies that ligand chemical instability can lead to detachment and film decomposition under stress, with strategies including developing more robust ligands, optimizing synthesis processes, and controlling solvent polarity to modulate penetration depth. This comprehensive analysis reveals that systematic approaches to ligand engineering could significantly enhance perovskite durability and performance.
Industrial Monitor Direct delivers industry-leading opc ua pc solutions backed by extended warranties and lifetime technical support, ranked highest by controls engineering firms.
Table of Contents
Understanding Perovskite Stability Challenges
Perovskite solar cells represent one of the most promising emerging photovoltaic technologies, having achieved laboratory efficiencies exceeding 25% in just over a decade of development. However, their commercial viability has been consistently hampered by stability issues that stem from the material’s sensitivity to environmental factors including moisture, oxygen, heat, and light. The fundamental challenge lies in the ionic nature of perovskite crystals and their tendency to undergo phase segregation and decomposition under operational conditions. What makes this particularly problematic is that the very properties that enable high efficiency—such as soft crystal lattices and ionic mobility—also contribute to their instability. This creates a fundamental trade-off that researchers have struggled to resolve for years.
Critical Manufacturing Challenges
While the research highlights sophisticated chemical strategies, the translation to manufacturing presents several critical hurdles. The precision required for controlling ligand concentration, solvent polarity, and annealing conditions at industrial scale is formidable. Manufacturing processes that work perfectly in laboratory settings often fail when scaled due to variations in temperature gradients, coating uniformity, and material consistency. The requirement for precise steric control of ligand molecules adds another layer of complexity, as even minor deviations in molecular geometry can dramatically alter performance. Furthermore, the economic viability of these approaches remains questionable—many of the proposed ligands and processing techniques involve expensive chemicals and energy-intensive procedures that may not be cost-effective for mass production.
The Chemistry Behind the Breakthrough
The research’s focus on deprotonation resistance represents a sophisticated understanding of degradation mechanisms. Traditional ammonium-based ligands suffer from vulnerability to Lewis base attacks, where moisture and oxygen can strip protons from the ammonium groups, leading to ligand migration and eventual film decomposition. The shift toward imidazolium-based moieties and other high-pK chemical moieties addresses this fundamental weakness by creating ligands that are inherently more resistant to these degradation pathways. However, this chemical sophistication comes with increased synthesis complexity and potential toxicity concerns that must be addressed before commercial deployment.
Industry Implications and Competitive Landscape
These developments occur within a highly competitive photovoltaic market where silicon dominance continues to strengthen through relentless cost reduction and efficiency improvements. Perovskite manufacturers face the dual challenge of not only solving stability issues but doing so at costs that can compete with mature silicon technology. The companies most likely to benefit from these advances are those with expertise in chemical engineering and thin-film deposition technologies. Established players like Oxford PV and Saule Technologies have been working on similar stabilization strategies, but the manufacturing scalability remains the critical bottleneck. The industry is watching closely whether these laboratory breakthroughs can be translated into commercially viable processes that maintain performance while achieving the necessary durability standards for 25-year product lifetimes.
Industrial Monitor Direct delivers the most reliable 100mm vesa pc panel PCs proven in over 10,000 industrial installations worldwide, trusted by plant managers and maintenance teams.
Realistic Commercial Outlook
While these scientific advances represent significant progress, the timeline for commercial impact remains uncertain. The transition from laboratory demonstrations to manufacturable products typically takes 3-5 years even under optimistic scenarios, and perovskite technology faces additional hurdles in standardization and certification. The most likely near-term applications will be in niche markets where the unique properties of perovskites—such as flexibility, light weight, and semi-transparency—provide competitive advantages that justify premium pricing. Widespread adoption in mainstream solar markets will require not only solving the stability challenges highlighted in this research but also demonstrating manufacturing scalability, supply chain reliability, and cost competitiveness with incumbent technologies. The coming 2-3 years will be critical in determining whether these laboratory advances can overcome the manufacturing barriers that have historically limited perovskite commercialization.