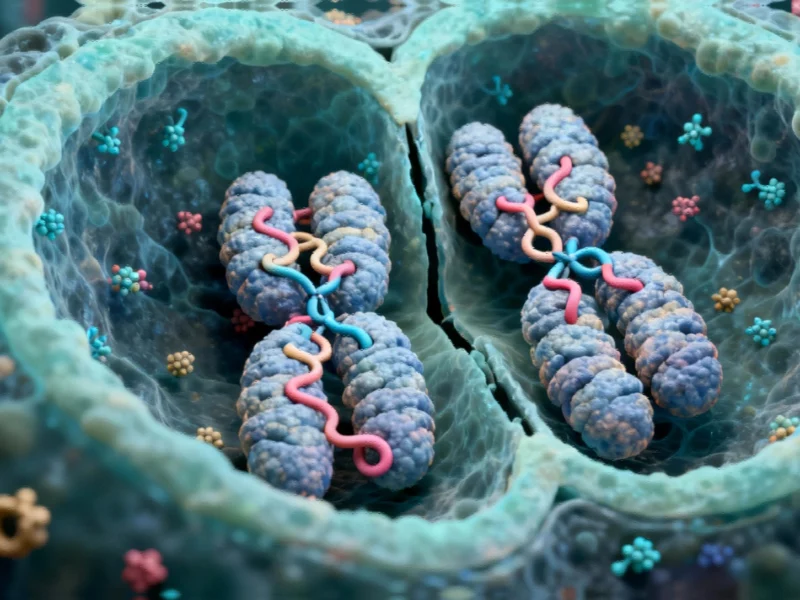
Rethinking Cell Division: Persistent Genome Loops Challenge Long-Held Biological Dogma
In a groundbreaking discovery that rewrites textbook understanding of cell biology, MIT researchers have revealed that dividing cells maintain crucial 3D genomic structures previously believed to completely disassemble during mitosis. This surprising finding, detailed in Nature Structural & Molecular Biology, demonstrates that tiny regulatory loops persist throughout cell division, potentially serving as a cellular memory system that carries gene regulation information from one generation to the next. The research builds upon earlier work documented at EAM Vision Direct’s coverage of persistent genome loops while revealing entirely new dimensions of this biological phenomenon.
“This study fundamentally changes how we understand mitosis,” says Anders Sejr Hansen, MIT associate professor of biological engineering and senior author of the study. “We’ve moved from thinking of cell division as a complete reset to recognizing it as a process that maintains crucial architectural elements of the genome.” The discovery helps explain how cells maintain their identity through countless divisions and represents a significant advancement in our understanding of genetic inheritance mechanisms.
The Technical Breakthrough Behind the Discovery
The research team achieved their breakthrough using Region-Capture Micro-C (RC-MC), a cutting-edge technique they developed in 2023 that provides 100 to 1,000 times greater resolution than previous genome mapping methods. Unlike conventional Hi-C mapping, which chops the genome into random fragments, RC-MC uses specialized enzymes to create uniformly sized fragments and focuses on specific genome regions, enabling unprecedented detail in 3D structural analysis.
This technological advancement follows similar progress in other scientific fields, such as the new telescope technology cutting through space noise that has revolutionized astronomical observations. In both cases, enhanced resolution has revealed previously invisible structures and patterns, opening new avenues for scientific exploration.
Microcompartments: The Unexpected Survivors of Cell Division
During their investigation, the researchers identified what they term “microcompartments” – tiny, highly connected loops that form when genetic regulatory elements called enhancers stick to gene promoters. These structures were initially discovered in non-dividing cells, but the assumption was that they would disappear during mitosis along with larger genomic structures known as A/B compartments and topologically associating domains (TADs).
Remarkably, the opposite proved true. “We went into this study thinking, well, the one thing we know for sure is that there’s no regulatory structure in mitosis, and then we accidentally found structure in mitosis,” Hansen recounts. Not only do microcompartments persist, but they actually strengthen during chromosome compaction in preparation for cell division.
This finding highlights how technological limitations can shape scientific understanding, much like how AI’s cargo cult problem demonstrates how superficial understanding can lead to flawed implementations of complex systems.
Solving the Mitosis Transcription Mystery
The persistence of microcompartments during cell division may explain a long-standing puzzle in cell biology: the brief spike in gene transcription that occurs near the end of mitosis. Since the 1960s, scientists believed transcription ceased completely during cell division, but studies in 2016 and 2017 revealed a transient burst of transcriptional activity.
The MIT team discovered that microcompartments are more likely to form near genes that experience this transcriptional spiking. As chromosomes compact during mitosis, enhancers and promoters are pushed closer together, facilitating the formation of these regulatory loops. “It almost seems like this transcriptional spiking in mitosis is an undesirable accident that arises from generating a uniquely favorable environment for microcompartments to form,” Hansen explains.
This accidental activation parallels challenges in other technological domains, such as the digital isolation issues arising from geopolitical conflicts, where systems behave in unexpected ways due to external pressures.
Implications for Cellular Memory and Disease
The discovery that regulatory loops persist through cell division suggests a potential mechanism for cellular memory. “This may help cells ‘remember’ interactions present in one cell cycle and carry it to the next one,” says Viraat Goel, the study’s lead author. The strengthening of these loops during chromosome compaction could serve as a bookmarking system that ensures daughter cells maintain the gene expression patterns of their parent cells.
This cellular memory system operates alongside other advanced biological mechanisms, similar to how expanding AI capabilities are creating more sophisticated computational systems. Both represent frontiers in understanding complex information processing systems.
Future Research Directions
The research team is now exploring how physical cell characteristics influence genome structure and gene regulation. “We are thinking about some natural biological settings where cells change shape and size, and whether we can perhaps explain some 3D genome changes that previously lacked an explanation,” Hansen says.
A key unanswered question involves how cells determine which microcompartments to maintain after division. “How does the cell then pick what are the microcompartments to keep and what are the microcompartments to remove when you enter G1, to ensure fidelity of gene expression?” Hansen questions. Understanding this selection process could reveal fundamental principles of cellular decision-making.
This research direction mirrors advances in materials science, where record-breaking chips are sidestepping Moore’s Law through innovative approaches to structural organization, demonstrating how physical arrangement can dramatically enhance functional capabilities.
Bridging Genome Structure and Function
The findings represent a significant step toward understanding how genome architecture influences gene expression. “The findings help to bridge the structure of the genome to its function in managing how genes are turned on and off, which has been an outstanding challenge in the field for decades,” Goel emphasizes.
Independent experts recognize the significance of this advancement. Effie Apostolou, associate professor of molecular biology at Weill Cornell Medicine (not involved in the study), notes that “this study leverages the unprecedented genomic resolution of the RC-MC assay to reveal new and surprising aspects of mitotic chromatin organization, which we have overlooked in the past using traditional assays.”
As research continues, these persistent genome loops may reveal new insights into developmental biology, cancer progression, and cellular aging, fundamentally changing our understanding of how genetic information is preserved and transmitted across cell generations.
Based on reporting by {‘uri’: ‘phys.org’, ‘dataType’: ‘news’, ‘title’: ‘Phys.org’, ‘description’: ‘Phys.org internet news portal provides the latest news on science including: Physics, Space Science, Earth Science, Health and Medicine’, ‘location’: {‘type’: ‘place’, ‘geoNamesId’: ‘3042237’, ‘label’: {‘eng’: ‘Douglas, Isle of Man’}, ‘population’: 26218, ‘lat’: 54.15, ‘long’: -4.48333, ‘country’: {‘type’: ‘country’, ‘geoNamesId’: ‘3042225’, ‘label’: {‘eng’: ‘Isle of Man’}, ‘population’: 75049, ‘lat’: 54.25, ‘long’: -4.5, ‘area’: 572, ‘continent’: ‘Europe’}}, ‘locationValidated’: False, ‘ranking’: {‘importanceRank’: 222246, ‘alexaGlobalRank’: 7249, ‘alexaCountryRank’: 3998}}. This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.